BRAM-COR ensures a clear answer to any request related to water treatments for pharmaceutical water production and any other critical water application (e.g. cosmetics, health-care, human and animal aliments). We apply a very focalised and precise approach and a wide range of specific knowledge. Our reverse osmosis CROS systems, are designed to produce purified water (PW) and water for injection (WFI), through a number of water treatment phases, based on specific feed water’s quality and on the specified production’s necessities.

Specifically, Mod plants. CROS with reverse osmosis technology for pharmaceutical use (Sanitary Reverse Osmosis Systems) produce PW in a highly convenient and efficient manner and, with downhill systems, an additional ultrafiltration and for WFI.
CROS operates through different water treatment phases, both related to the specific water quality and to the production necessities. The treatment phases which are needed to divide water from any organic substance, both with high or medium molecular weight, include:
While WFI needs downhill ultrafiltration, purified water, traditionally produced through reversed osmosis, is commonly produced through:
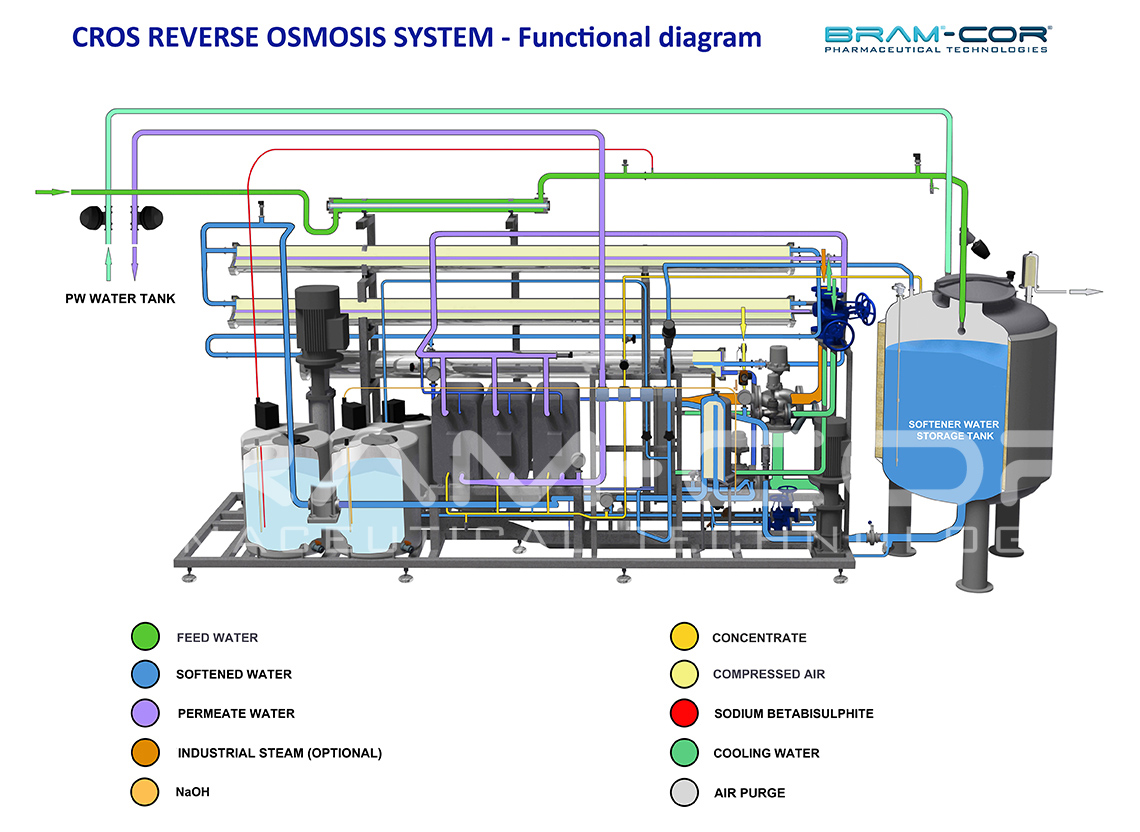
In each step of reversed osmosis (RO) water is treated through membranes composed by thin films, (TFC or TFM), which offer the highest retention of contaminants.
Two different flows are generated by RO systems: permeated, corresponding to the specific PW, and concentrated RO, which is recycled in the system to obtain a higher regain with a lower feed water expenditure. An additional stage in the RO process can be included to reduce water waste through the concentrated RO.
BRAM-COR’s COR permeation capacities ranges from 100 until 20000 litres per hour (l/h) developed according to the cGMP and FDA indications, which include:
Processes such as softening, decalcification, pre-filtration, degassing, nano-filtration, electro-deionisation, ozonation, UV treatment and microfiltration, can be considered in relation to feed water’s quality. In turn, water’s quality has to be compliant to the pharmacopeia’s quality levels if, for example, the water has to be used for the production of WFI.
Nevertheless, the WFI production with reversed osmosis systems require particular attention, unless the feed water is truly excellent, since RO does not include a change in the state of the substance (e.g. from liquid to gas, i.e. a natural microbiological barrier). Through the availability of technological membranes which are more advanced in ultrafiltration, we are still able to provide WFI in cold water, a process which enables to fulfil the range of required parameters (USP, EP, JP…).
Our RO + ultrafiltration systems reach an excellent WFI quality; nevertheless, once produced, any RO system requires constant monitoring related to the degradation of the membrane, in function of preventing the creation of eventual biofilm or micro charges, which is possible through disinfection and periodical validations. If there are no roadblocks which undermine a constant monitoring, which should also concern the distribution circuits, the reversed osmosis systems can offer a significant advantage, reducing energy expenditure and, hence, production costs.
For further information see pharmaceutical-reverse-osmosis-systems.com
