We provide complete design, engineering, construction, start-up of new pharmaceutical facilities, even assisting the Clients through scouting activities of potential Know How Licensor, transferring the Know How, providing Validation Master Plans and Standard Operating Procedures, validating, aligning industrial processes to URS and regulatory requirements.
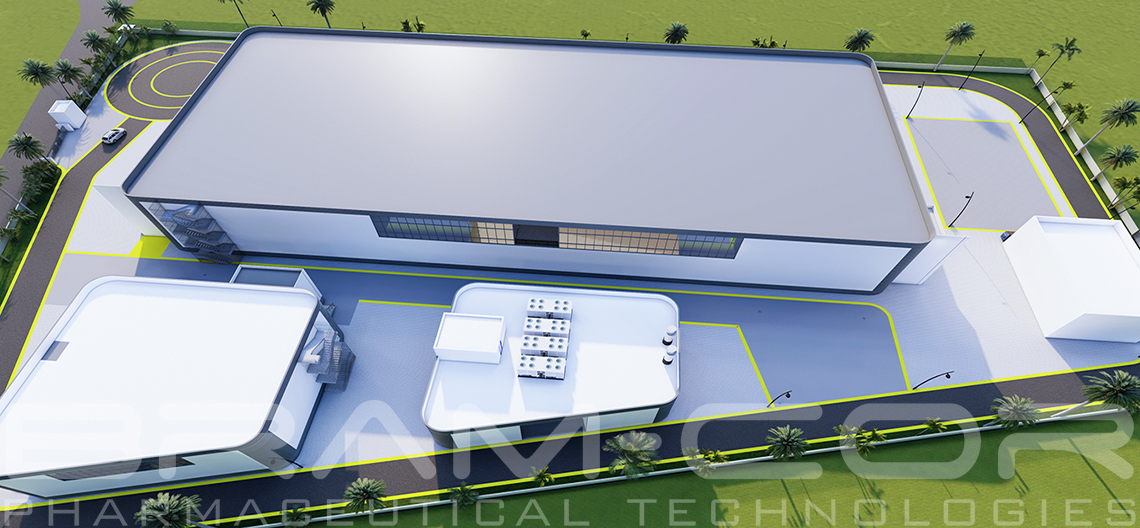
For more details about turnkey plants for IV Fluids and Parenteral Solutions, you can see, too: ivfluids-parenteralsolutions.com
For more details about BRAM-COR Turnkey project, please visit us at pharmaceutical-turnkey.com
To request information regarding turnkey projects, please write to: international@bram-cor.com
BRAM-COR activities are regulated by BRAM-COR Corporate Quality System and described in the Quality Manual, detailed by the relevant Standard Operating procedures. The following list defines main BRAM-COR certified Quality System Procedures and main Operating Instructions regulating Design, Construction, Testing and Project Managing activities, also for Turnkey Projects.
|
Activity |
Procedure |
Title |
|
Design |
PO 73_01 |
Design Management |
|
Purchase |
PO 74_01 |
Purchase Management |
|
PO 74_02 |
Supplier’s Assessment |
|
|
Production and Installation |
PO 75_01 |
Job Management |
|
PO 75_02 |
Customer’s Properties |
|
|
PO 75_03 |
Assistance |
|
|
PO 75_04 |
Identification and Traceability |
|
|
PO 75_05 |
Process Control |
|
|
PO 75_06 |
Delivery and Commissioning |
|
|
PO 75_08 |
Machines Construction |
|
|
PO 75_09 |
Sanitary Piping |
|
|
PO 75_10 |
Technical Documentation |
|
|
Inspection & Monitoring |
PO 76_01 |
Measuring Instruments Management |
|
PO 83_01 |
CAPA Management |
The standards of good manufacturing practice (cGMP) require special attention to risk assessment and verification procedures: “… it is requirement of good manufacturing identify the activities of validation necessary to demonstrate control critical aspects of particular operations. The significant changes made to installations, equipment and processes, which may affect product quality, should be validated. A procedure for risk assessment should be used to determine the scope and extent of validation.” The Validation Master Plan serves to make sure that all equipment, procedures, that may affect the quality or integrity or effectiveness of the product, are validated; it contains the general principles which comply during the validation task, and plans activities to be carried out for this purpose.
Below: a typical process flow-chart for infusional bags production
1. Basic Engineering 2. Detailed Engineering 3. Design Qualification 4.Inlet Water Pretreatment Plant 5. Pharmaceutical Water Systems (Softened, Purified and Distilled Water) 6. Pharmaceutical Processing and Solution Preparation Systems 7. Pharmaceutical Forming, Filling, Inspecting, Packaging lines 8. Clean Rooms 9. Epoxy coating of the floors 10. HVAC and air treatment plant 11. Autoclave 12. Pure Steam Generator and PS circuit 13. Laboratories of Analysis (Microbiological / Chemical) 14. Site Master Plan 15. Validation Master Plan 16. Installation 17. Training 18. Start up 19. Technical Files & Documentation 20. IQ/OQ 21. PQ Protocols 22. Validation at Site 23. Standard Operating Procedures 24. Initial Know How Transfer 25. GMP pre Audit 26. Spare parts for n years

LVP (Large Volume Parenterals) in PVC or PP bags
LVP in Glass or Plastic Bottles
SVP (Small Volume Parenterals) in Glass or Plastic Vials and Ampoules
Pre-filled Syringes
IV products (bags, glass and plastic bottles)
Blood bags (LVP bags in PVC)
Dialysis concentrate solutions and powders (Canisters, Bags, Cartridges)
Plasma Fractionation
Biotechnological Plants
Multipurpose Pharmaceutical Plants
Procurement of Licensors and Know How Transfers
Conceptual Design and Design Qualification (DQ)
Detailed Engineering
Validation Master Plan & S.O.P.
Equipment Construction & Procurement
Factory Acceptance Test (FAT)
Shipment and Installation
Site Acceptance Test (SAT)
Standard & Validation Documentation (IQ/OQ/PQ Protocols)
Training
Commissioning, Calibration & Start up
Regulatory Suppor
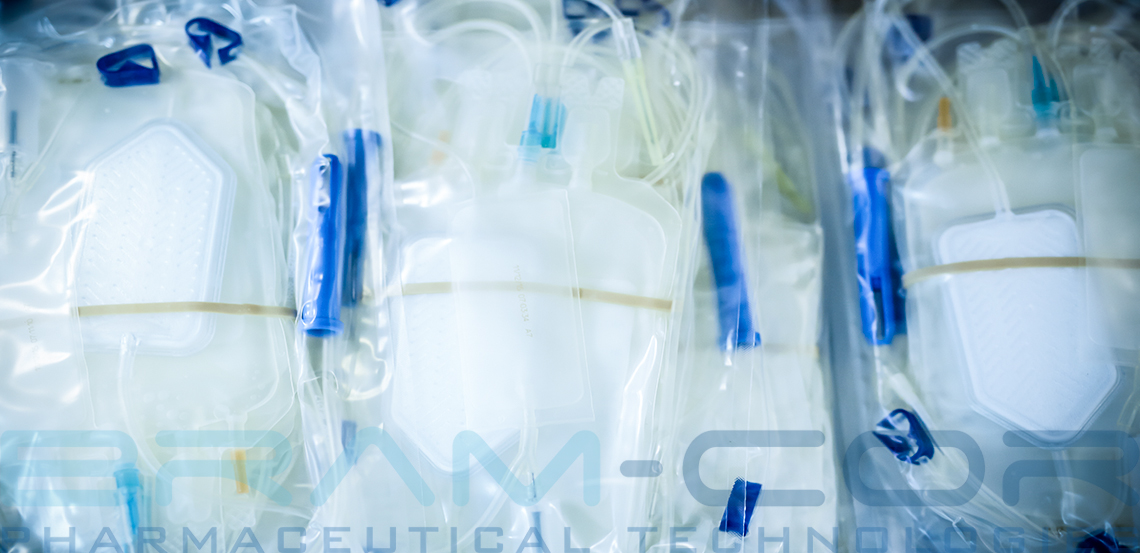
Sodium Bicarbonate, Peritoneal Dialysis, Isotopes of Calcium, Glucose, Dialysis Fluid, Hemodialysis, Lactic Acid, Peritonitis, Ultrafiltration, ….
Read all
This service ensures small or medium size pharmaceutical brands to reach…
Read all
Bram-Cor operations include turnkey plants for pharmaceutical veterinary applications. Leveraging our expertise in pharmaceutical process engineering and GMP-compliant facility design...
Read all